Ozone Therapy for Helicobacter pylori
At a Glance:
For a complete overview of conditions, see what diseases does ozone therapy treat.
- Helicobacter pylori (H. pylori) is a common stomach infection that affects approximately 50% of people worldwide. It can inflame the stomach lining and reduce stomach acid.
- It's a hardy bacterium that's very difficult to eradicate, requiring 3-4 drugs, and is becoming increasingly antibiotic-resistant. After each round of treatment, it can often recur due to host weakness, reinfection from household members and food items, or re-emerge from dormancy in the stomach.
- Conventional H. pylori treatment, which typically involves the use of at least two antibiotics, a stomach acid-reducing drug, and possibly a bismuth compound, can have severe side effects on the microbiome and overall health.
- Low stomach acid, resulting from H. pylori infection or the use of acid-reducing drugs, can increase susceptibility to other diseases, small intestinal bacterial overgrowth, and fungal overgrowths, as well as dysbiosis. The low stomach acid can cause deficiencies in vitamin B12, iron, and several other minerals.
- Ozone therapy can enhance the host's terrain to combat H. pylori, while oral ozone oil and water ingestion may help eradicate the bacterium or disrupt its biofilms. Ozone therapy also supports the regeneration of the stomach lining with little side effects. When combined with nutrients such as zinc, L-carnosine, and vitamin A, this integrated approach may be more effective than conventional therapy alone.
Helicobacter pylori (H. pylori) is a common stomach infection that can cause low stomach acid, nutrient deficiencies, and many other ripple effects throughout the body. It can be a notoriously difficult infection to treat, particularly with increasing antibiotic resistance. This is where ozone therapy can help.
This article covers H. pylori, host factors that make specific individuals susceptible, the shortcomings of current treatments, and how ozone therapy can help, whether used alone or in combination with other treatments.
What Is H. pylori, and Why Is It Problematic?
H. pylori is a spiral-shaped, gram-negative bacterium uniquely adapted to survive and thrive within the acidic environment of the human stomach [1].
Although many people can harbor H. pylori in their stomach or duodenum (the first part of the small intestine) without any negative effects, it is far from benign.
In susceptible individuals, chronic H. pylori infections disrupt stomach function, change certain aspects of the immune response, and set the stage for chronic gut issues.
More than half the world’s population is infected with H. pylori, though prevalence varies dramatically by region and socioeconomic status. Rates exceed 70% in parts of Asia, Africa, and South America [2].
In the United States, the prevalence is around 30%, with higher rates among older adults, immigrants, and communities with poor sanitation.
H. pylori is particularly problematic because it can be stealthy and persistent. It burrows into the mucosal layer of the stomach, adhering tightly to epithelial cells using surface proteins.
After adhering, it secretes the enzyme urease, which neutralizes local gastric acid to create a more hospitable microenvironment. This allows the bacteria to evade the acidic barrier that typically protects against any microbial colonization.
Over time, H. pylori can stimulate the release of pro-inflammatory cytokines and reactive oxygen species. The bacteria can also infiltrate immune cells to cause cell deaths or more pathology [3], [4], [5]. These lead to chronic stomach inflammation (gastritis), which, in some individuals, progresses to more serious conditions like [6]:
- Peptic ulcers
- Atrophic gastritis
- Mucosa-associated lymphoid tissue (MALT) lymphoma
- Gastric adenocarcinoma
This bacterium is now classified as a Group 1 carcinogen by the World Health Organization, which places it in the same group as ionizing radiation, tobacco, alcohol, and cancer-causing (oncogenic) viruses [7].
H. pylori’s disease-causing ability (pathogenicity) is enhanced by a set of well-characterized virulence factors [8]:
- Urease breaks down urea into ammonia, buffering the bacterium against acid.
- CagA (cytotoxin-associated gene A): a protein injected into host cells that disrupts normal signaling, linked to increased inflammation and cancer risk.
- VacA (vacuolating cytotoxin A): disrupts cell membranes and mitochondrial function, promoting epithelial cell injury.
- Adhesins: allow tight binding to host tissues, making eradication more difficult.
In short, H. pylori is not simply a stomach bug; it is a chronic intruder that:
- Hijacks the gastric environment
- Manipulates host immunity
- Increases risk for serious disease
Left unchecked, it can linger for decades—often without symptoms—until pain, indigestion, fatigue, nutrient deficiencies emerge, or more permanent damage appears.
Conventional Treatments of H. pylori and Their Shortfalls
Standard treatment for H. pylori infection typically involves triple or quadruple therapy, which is a combination of [9]:
- At least two antibiotics (most often clarithromycin, amoxicillin, or metronidazole), to eradicate the bacteria.
- One proton pump inhibitor (PPI), which suppresses stomach acid to provide an optimal environment for the antibiotics to work and protect the inflamed stomach issues from stomach acid.
- A bismuth-containing compound, which helps protect the stomach lining, has antibacterial properties, and boosts treatment effectiveness.
These treatments, while effective, can have some downsides:
- Adverse impacts on the gut flora, and thus overall long-term health.
- Antibiotic resistance and reduced effectiveness.
Clarithromycin resistance alone now exceeds 20% in many regions, significantly compromising the effectiveness of first-line therapies [10].
Treatment success rates, which were once reliably above 90%, are now 60% and 80%, depending on geographic location, prior exposure, and adherence. The trend is clear: H. pylori is adapting, and conventional regimens are becoming less effective.
3) Possible recurrence, even after eradication.
Reinfection or recrudescence (incomplete eradication that later resurfaces) can occur, particularly in high-prevalence areas or where gastrointestinal health is already compromised.
The bacteria can survive for hours or days on surfaces and in food, even in refrigerated or pasteurized foods [11], [12], [13]. Additionally, partners or family members can harbor the bacteria and become a source of reinfection.
H. pylori can shift from its traditional spiral shape to a spherical (coccoid) form under environmental stress, helping it survive in harsh environments like water or the stomach [14]. This makes it harder to detect and eliminate using standard culturing methods.
4) Increased susceptibility to other gut infections
PPI can increase susceptibility to other gut infections such as small intestinal bacterial overgrowth (SIBO), parasites, and dysbiosis [15]. The drug can also cause rebound acid hypersecretion after usage stops, while prolonged use can cause a deficiency in vitamin B12, iron, and many other minerals [16]:
These consequences are especially problematic in individuals already suffering from fatigue, gut dysfunction, or in an immunocompromised state.
5) Other side effects
These can be severe, including nausea, diarrhea, bloating, and even the onset of new symptoms [17].
Some patients cannot tolerate the treatments, while others find that even after therapy, their digestive symptoms persist. This shows the limitations of an approach that kills the bacterium but fails to restore overall balance in the gut.
The approach that focuses on eradication rather than the person’s susceptibility tends to fail. This leaves behind a stomach terrain vulnerable to future infections or recurrences.
Some patients prefer gentler, natural protocols, such as herbs and probiotics, or incorporate natural remedies to support the terrain.
Ozone Therapy for H. pylori – The Evidence
An animal study used an indomethacin-induced acute gastric ulcer model in rats to evaluate the protective effects of oxygen-ozone therapy on gastric ulcer formation and oxidative damage. Ozone was compared to lansoprazole, a standard proton pump inhibitor (PPI) [18].
The study divided 28 male Wistar rats into four groups: control, sham (indomethacin-only), ozone-treated, and lansoprazole-treated. The ozone group received 5 mL of an oxygen-ozone gas mixture before the induction of ulcers with indomethacin. The Lansoprazole group received 30 mg/kg orally.
Results showed that:
- The sham group developed the largest ulcers (71.47 ± 17.08 mm²).
- The ozone group had significantly smaller ulcers (20.79 ± 12.88 mm²), while the lansoprazole group had the smallest (5.80 ± 3.85 mm²).
- Ozone therapy significantly reduced oxidative markers (MPO, MDA, 8-OHdG) and increased antioxidant defenses (tGSH, SOD), outperforming lansoprazole.
Although lansoprazole had better macroscopic ulcer reduction, ozone provided more robust antioxidant and cellular protection, suggesting a complementary or preventive role in gastric health management.
While this study used non-steroidal anti-inflammatory drugs (NSAIDs), Indomethacin, to induce ulcers, the mechanisms of injury—oxidative stress, epithelial damage, and inflammation—are shared with H. pylori-induced mucosal injury.
H. pylori infection increases oxidative stress and impairs mucosal defenses, contributing to gastritis and ulceration in a manner comparable to NSAIDs.
The demonstrated ability of ozone therapy to boost antioxidant defenses and limit cellular damage supports its potential as a non-antibiotic adjunct or preventive therapy in H. pylori-associated gastric ulcers.
Rectal Insufflation
Currently, no published studies exist on the use of ozone rectal insufflation in the treatment of H. pylori. However, rectal insufflation may support the immune system and gut flora during the treatment of H. pylori.
Drinking Ozone Water and Ingesting Oral Ozone Oil
Case series 1 evaluated the effectiveness of a 90-day ozone-based treatment protocol as a standalone therapy for eradicating H. pylori in 21 patients with a confirmed infection [19]:
- Ozonated saline IV was administered every 15 days (ozonated saline solution: 200 mL, 2 μg/kg over 10 minutes).
- Oral ozone therapy consisted of 1 mL of ozonated olive oil (600 meqO₂) taken 20 minutes before meals, daily for 90 days.
- Post-treatment endoscopy was performed in patients who were previously positive in assessing eradication.
Treatment protocols showed that:
- Endoscopic follow-up showed H. pylori eradication in 11 of the 14 infected patients (78.6%).
- Clinical improvement was observed in 85% of all patients, regardless of H. pylori status. Most reported resolution of symptoms such as bloating, nausea, and reflux, with burping being the only occasional residual symptom.
- No adverse events or complications were reported, and the protocol was well tolerated.
A randomized clinical trial of 132 H. pylori-positive patients evaluated whether drinking ozonated tri-distilled water combined with standard triple therapy (esomeprazole, amoxicillin, clarithromycin) would improve H. pylori eradication rates and duodenal ulcer healing, compared to triple therapy alone [20].
Patients were divided into four groups:
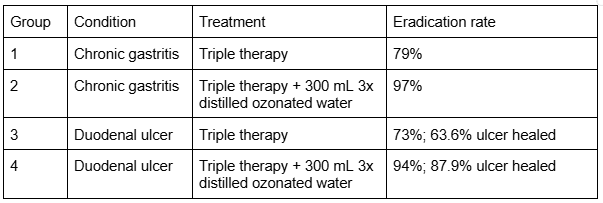
The study also found:
- No adverse effects were observed from drinking ozonated water in H. pylori-positive chronic gastritis and duodenal ulcer patients.
- No significant changes were noted in carcinoembryonic antigen (CEA) levels, suggesting safety regarding mucosal integrity.
Combining ozonated tri-distilled water with standard triple therapy significantly improves both H. pylori eradication and duodenal ulcer healing, compared to antibiotics alone.
Ozone’s broad antimicrobial and mucosal-supportive effects, along with its safety and simplicity, make it a compelling adjunct in gastrointestinal protocols, particularly as antibiotic resistance continues to rise globally.
IV Ozone Therapy
Currently, no published studies exist on the use of intravenous (IV) ozone therapy in the treatment of H. pylori. However, as a systemic treatment, IV ozone therapy may support the immune system during standard or oral ozone oil treatment.
Ingesting Ozone Oil
An in vivo animal study used an ethanol-induced gastric ulcer model in rats to investigate the gastroprotective effects of ozonated sunflower oil (OSO) [21].
Rats received oral OSO via intragastric cannula at 4, 12, or 24 mg/kg/day for four days. One hour after the final dose, absolute ethanol was administered to induce gastric mucosal injury.
OSO treatment resulted in:
- Significantly less gastric ulcer formation compared to ethanol-only controls.
- No significant change in lipid peroxidation, suggesting that OSO’s protective effect was not due to direct suppression of lipid damage.
- Superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity increased significantly in OSO-treated groups, indicating enhanced endogenous antioxidant defense.
Pretreatment with ozonated sunflower oil offers protective effects against ethanol-induced gastric injury, mostly by upregulating key antioxidant enzymes that neutralize damaging reactive oxygen species. The effect was dose-dependent and well-tolerated in the animal model.
Although the ulcer model here is ethanol-induced, the mechanisms of gastric injury—oxidative stress, mucosal inflammation, and epithelial disruption—overlap significantly with those caused by H. pylori.
H. pylori triggers sustained reactive oxygen species (ROS) production and suppresses local antioxidant systems, weakening the gastric barrier over time.
The ability of ozonated oil to stimulate antioxidant enzymes, like SOD and GSH-Px, may represent a shared therapeutic mechanism in protecting against both chemically and microbially induced gastric injury.
These findings reinforce the role of ozonated oils as mucosal protectants and modulators of redox balance, supporting their application in H. pylori-associated gastritis and ulceration.
Ingredients and products that may be more effective to treat H. pylori
L-Carnosine
L-carnosine has received increasing attention for stomach-protecting, antioxidant, and anti-inflammatory properties [22]. As such, L-carnosine complements ozone therapy in managing H. pylori-associated stomach issues.
One of L-carnosine’s most relevant features is its ability to bind and neutralize reactive carbonyl species, limiting protein and lipid oxidation. Ozone’s mild oxidative preconditioning effects also target these mechanisms..
This shared biochemical activity suggests that L-carnosine may buffer oxidative stress while allowing ozone’s signaling benefits to proceed unimpeded.
Additionally, L-carnosine has demonstrated the ability to:
- Stabilize gastric epithelial cells
- Reduce inflammatory cytokine release
- Promote tissue repair following ulceration
In experimental models of stomach injury caused by NSAIDs and ethanol, L-carnosine preserved mucosal integrity, reduced lesion size, and improved histological architecture.
Particularly, zinc mixed with L-carnosine is an effective adjunct to H. pylori eradication therapy, improving ulcer healing and decreasing inflammation in the gastric mucosa [23].
Although studies combining ozone and carnosine are limited, their mechanistic complementarity—ozone-stimulating antioxidant systems like SOD and GSH, while carnosine neutralizing harmful byproducts of oxidative stress—points toward a potential synergistic role.
In gastric formulations, especially those designed to linger at the mucosal surface, L-carnosine may enhance ozone’s therapeutic reach.
Zinc
Zinc is a trace element crucial for gastric mucosal health, immune function, and antioxidant defense, all of which are disrupted in H. pylori infections. Those with H. pylori infections tend to have low levels of zinc in the serum, making it even harder for them to fight off infection [24]. The lower levels of zinc in the stomach lining correlate with more severe H. pylori infections [25].
Zinc serves as a cofactor for numerous biological processes and gene readouts. Notably, zinc supports the following factors in protecting the stomach tissue from acidity:
- Tight junction integrity
- Epithelial repair
- Immune defense and inflammation regulation
A systematic review of three randomized controlled trials evaluated the safety and efficacy of adding zinc carnosine to standard triple therapy for H. pylori eradication [26].
Analysis showed that:
- Triple therapy and zinc carnosine significantly improved eradication rates (p = 0.003).
- There was no significant difference in adverse events (p = 0.85).
The addition of zinc to antibiotic regimens reduced pro-inflammatory cytokines, such as IL-1β and TNF-α, while protecting gastric epithelial cells from programmed cell death.
From a mechanistic perspective, zinc’s role complements ozone therapy exceptionally well. Ozone initiates a mild, transient oxidative stress that activates endogenous antioxidant systems, such as glutathione and superoxide dismutase.
Zinc acts as a cofactor for these enzymes, meaning its presence is essential for their optimal function. Zinc also limits the adhesion of H. pylori to gastric cells, a critical early step in the infection process. So, zinc and ozone work together to modulate oxidative damage, support epithelial regeneration, and restore inflammatory signaling..
A randomized controlled clinical trial evaluated whether a modified bismuth quadruple therapy, enhanced with zinc carnosine, is more effective than standard triple therapy (TT) in eradicating H. pylori infection in dyspeptic patients [27].
Researchers randomized 92 patients with confirmed H. pylori infection into two treatment groups:
- Triple Therapy (TT): Esomeprazole 40 mg, amoxicillin 1 g, and clarithromycin 500 mg, all taken twice daily for 14 days. This group experienced a 69.6% eradication rate.
- Modified Bismuth Quadruple Therapy: TT plus bismuth subcitrate 240 mg and zinc carnosine 75 mg, both taken twice daily for 10 days. This group experienced a 93.5% eradication rate.
Aside from occasional dizziness, the enhanced regimen showed no major increase in adverse effects.
Vitamin A
Vitamin A is a crucial vitamin for the structural and functional integrity of mucosal surfaces throughout the body, including the stomach lining [28]. It works mainly as a cofactor for various gene readouts.
Within the context of H. pylori infection and gastric injury, vitamin A’s relevance becomes clear. The bacteria compromise the gastric lining by:
- Inducing chronic inflammation
- Disrupting epithelial tight junctions
- Impairing mucus secretion
Vitamin A directly counters these effects by [29], [30]:
- Stimulating the turnover and repair of epithelial cells, which helps maintain the integrity of the gut lining.
- Enhancing the production and secretion of protective mucus, which acts as a barrier against harmful microbes and irritants.
- Modulating local immune responses, including dendritic cells, helper T cells, and secretory IgA production [31].
Vitamin A deficiency can exacerbate gut inflammation, while supplementation supports faster repair of damaged gastric tissue and promotes the expression of protective mucins.
When combined with ozone therapy, vitamin A may synergistically enhance mucosal repair and reduce the risk of ulceration or recurrence.
While no large-scale trials combine vitamin A with ozone for H. pylori, their overlapping actions suggest they could work well together to protect the stomach lining and support immunity.
Vitamin D
Vitamin D is best known for its role in calcium metabolism, but its functions extend far beyond bone health, including immune regulation, mucosal barrier health, and antimicrobial activities.
Those with vitamin D deficiency are more likely to catch infections, struggle to fight them off, and experience long-term inflammatory symptoms [32]. Therefore, H. pylori colonization and poorer eradication outcomes could be related to low vitamin D levels.
Vitamin D helps by:
- Increasing cathelicidin (LL-37) and β-defensins from the epithelial cells. These are small proteins that can disrupt bacterial membranes, including those of H. pylori.
- Modulating stomach inflammation that can contribute to stomach ulcers.
- Supporting tight junction integrity and stomach barrier function.
In this way, vitamin D complements ozone therapy’s ability to modulate oxidative stress, reduce inflammation, and stimulate epithelial repair.
While ozone improves local antioxidant enzyme activity (e.g., SOD, GSH), vitamin D supports systemic immune balance, thereby reducing the risk of collateral inflammation during bacterial turnover.
Though direct studies pairing vitamin D with ozone therapy for H. pylori are limited, their complementary biological effects make them an ideal combination for restoring gastric health.
Vitamin D repletion may help reduce recurrence rates and improve overall treatment outcomes, especially in individuals with known deficiencies.
An animal study investigated the role of vitamin D3 (1,25-dihydroxyvitamin D3) in controlling H. pylori infection and identified the role of the VDR-cathelicidin antimicrobial peptide (CAMP) pathway in gastric mucosal immunity [33].
Wild-type (VDR⁺/⁺) and VDR-knockdown (VDR⁺/⁻) mice were infected with the H. pylori SS1 strain via intragastric administration. Following infection confirmation, the mice were treated orally with various doses of vitamin D3.
Vitamin D treatment showed that:
- VDR-deficient mice (VDR⁺/⁻) showed greater susceptibility to H. pylori and lower baseline expression of both VDR and CAMP in the stomach.
- H. pylori infection slightly upregulated VDR and CAMP in gastric tissues.
- D3 treatment significantly increased VDR and CAMP expression in infected mice and led to a dose-dependent reduction in H. pylori colonization.
- D3 did not cause hypercalcemia or alter phosphorus levels, showing a favorable safety profile at the administered doses.
Oral vitamin D3 supplementation enhanced gastric mucosal defenses against H. pylori through the upregulation of the VDR–CAMP signaling axis.
By increasing local antimicrobial peptide expression, vitamin D3 not only reduces bacterial colonization but also strengthens mucosal immunity in a mechanism that is independent of calcium metabolism.
Molybdenum
Though less commonly discussed in gut health than zinc or vitamin A, molybdenum plays a subtle yet significant role in maintaining gut homeostasis, especially regarding oxidative stress and microbial byproducts [34].
As a trace mineral cofactor, molybdenum is essential for several detoxifying enzymes, most notably sulfite oxidase, which converts sulfites to harmless sulfates in the body [35].
This pathway becomes especially important in the stomach, where microbial overgrowth, inflammation, or bacterial die-off can increase the local burden of sulfur-containing metabolites [36].
When ozone therapy or any antimicrobial treatment is introduced, it may lead to the oxidation and breakdown of pathogenic organisms, potentially releasing toxic intermediates such as aldehydes, amines, and sulfites [37], [38].
By supporting enzymes like aldehyde oxidase and xanthine oxidase [39], [40], molybdenum helps process these compounds. It may reduce the die-off burden that can temporarily irritate the mucosa or cause systemic symptoms during ozone-based treatment.
Though direct studies combining molybdenum and ozone in H. pylori treatment are lacking, molybdenum plays important roles in the body, including:
- Sulfite clearance
- Enzyme activation
- Metabolic detoxification
Therefore, molybdenum may help buffer the oxidative load induced during bacterial turnover.
For patients prone to sluggish detoxification, histamine sensitivity, or food intolerance—all common problems linked to chronic H. pylori infection or post-eradication dysbiosis (gut disruption)—molybdenum offers a gentle layer of support.
When paired with ozone’s redox signaling and epithelial repair benefits, molybdenum may help ensure that progress is smoother, more complete, and less reactive.
Herbal Antimicrobials and Mucosal Barrier Support
Some patients prefer herbs and probiotics over conventional therapies due to lower side effects and less disruption of the gut flora. In some cases, it may be beneficial to combine some of these treatments with ozone therapy or conventional therapy.
It’s crucial to work with an experienced practitioner to minimize side effects, prevent harmful drug interactions, and minimize the chances of recurrences or antibiotic resistance.
Herbal antimicrobials may include, but are not limited to, (not an exhaustive list) [41], [42]:
- Mastic gum
- Matula tea
- Olive leaf extract
- Deglycyrrhizinated licorice [43]
- Garlic extract [44]
- Oil of oregano
- Monolaurin
Natural stomach soothers may include [42]:
- Cabbage juice
- L-glutamine
- Aloe vera juice
- Slippery elm
- Marshmallow root
Conclusion
H. pylori is a remarkably common gut infection that can be very difficult to eradicate. When left untreated, it can cause a range of effects throughout the gut and, thus, all aspects of health.
Ozone therapy and the right nutritional support can improve H. pylori treatment outcomes, whether used alone or in combination with other treatments. Currently, there is more evidence for drinking ozone water and consuming ozone oil than for systemic treatments to eradicate H. pylori.
1 Parikh, N. S. and Ahlawat, R. (2023) Helicobacter Pylori. In StatPearls [Internet], StatPearls Publishing
2 Chen, Y.-C., Malfertheiner, P., Yu, H.-T., Kuo, C.-L., Chang, Y.-Y., Meng, F.-T., et al. (2024) Global prevalence of Helicobacter pylori infection and incidence of gastric cancer between 1980 and 2022. Gastroenterology 166, 605–619
3 Sugimoto, M., Furuta, T. and Yamaoka, Y. (2009) Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori. J Gastroenterol Hepatol 24, 1725–1732
4 Sah, D. K., Arjunan, A., Lee, B. and Jung, Y. D. (2023) Reactive oxygen species and infection: A comprehensive review of their roles in gastric cancer development. Antioxidants (Basel) 12
5 Tsai, H.-F. and Hsu, P.-N. (2010) Interplay between Helicobacter pylori and immune cells in immune pathogenesis of gastric inflammation and mucosal pathology. Cell Mol Immunol 7, 255–259
6 Malfertheiner, P., Camargo, M. C., El-Omar, E., Liou, J.-M., Peek, R., Schulz, C., et al. (2023) Helicobacter pylori infection. Nat Rev Dis Primers 9, 19
7 American Cancer Society (2023) Known and probable human carcinogens. American Cancer Society. (Accessed: 23 June 2025).
8 Baj, J., Forma, A., Sitarz, M., Portincasa, P., Garruti, G., Krasowska, D., et al. (2020) Virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 10
9 UpToDate (2024) First-line treatment regimens for H. pylori infection in treatment-naïve adults (Accessed: 19 June 2025).
10 Thung, I., Aramin, H., Vavinskaya, V., Gupta, S., Park, J. Y., Crowe, S. E., et al. (2016) Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther 43, 514–533
11 Kramer, A., Schwebke, I. and Kampf, G. (2006) How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 6, 130
12 Poms, R. E. and Tatini, S. R. (2001) Survival of Helicobacter pylori in ready-to-eat foods at 4 degrees C. Int J Food Microbiol 63, 281–286
13 Quaglia, N.C., Dambrosio, A., Normanno, G., Parisi, A., Firinu, A., Lorusso, V. & Celano, G.V. (2007) Survival of Helicobacter pylori in artificially contaminated ultrahigh temperature and pasteurized milk. Food Microbiol. 24, 296–300.
14 Loke, M. F., Ng, C. G., Vilashni, Y., Lim, J. and Ho, B. (2016) Understanding the dimorphic lifestyles of human gastric pathogen Helicobacter pylori using the SWATH-based proteomics approach. Scientific Reports, Nature Publishing Group 6, 1–8
15 Fujimori, S. (2015) What are the effects of proton pump inhibitors on the small intestine? World J Gastroenterol 21, 6817–6819
16 Maideen, N. M. P. (2023) Adverse Effects Associated with Long-Term Use of Proton Pump Inhibitors. Chonnam Medical Journal 59, 115
17 Hafeez, M., Qureshi, Z. A., Khattak, A. L., Saeed, F., Asghar, A., Azam, K., et al. (2021) Helicobacter Pylori eradication therapy: Still a challenge. Cureus 13, e14872
18 Bicer, S., Gursul, C., Demiryilmaz, I., Demiryilmaz, İ., Aydin Peker, N., Eken, H., Cimen, O., Demirtas, L. & Cakarli, S. (2016) Gastroprotective effect of oxygen-ozone therapy in the model of indomethacin-induced acute gastric ulcer in rats. Int. J. Clin. Exp. Med. 9, 22126–22133.
19 Rivera Soto, M. A., Romo-Vázquez, C. A. & Weber-Chuliá, N. (2018) Endoscopy evidence; H. pylori infection ozonetherapy treated, Mexican cases report. Revista Española de Ozonoterapia 8, 171–179.
20 Cheng, X., Wang, J., Zhao, H., Huang, S., Zeng, D., Feng, Y., Xu, Y., Fan, Z., Yue, Q., Wu, Z. & Cheng, J. (2016) Drinking ozonated tri-distilled water increases Helicobacter pylori eradication and promotes healing of duodenal ulcer lesions. Cronicon Open Access.
21 Zamora Rodríguez, Z. B., González Alvarez, R., Guanche, D., Merino, N., Hernández Rosales, F., Menéndez Cepero, S., et al. (2007) Antioxidant mechanism is involved in the gastroprotective effects of ozonized sunflower oil in ethanol-induced ulcers in rats. Mediators Inflamm 2007, 65873
22 Jukić, I., Kolobarić, N., Stupin, A., Matić, A., Kozina, N., Mihaljević, Z., et al. (2021) Carnosine, small but mighty-prospect of use as functional ingredient for functional food formulation. Antioxidants (Basel) 10
23 Mahmood, A., FitzGerald, A. J., Marchbank, T., Ntatsaki, E., Murray, D., Ghosh, S., et al. (2007) Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut 56, 168–175
24 Abdelkader, N. A., Abdelhakam, S. M., Hamed, A. M., Saad, W. E. & Ibrahim, W. A. (2016) Peptic ulcer disease and Helicobacter pylori infection: does serum zinc level play a role? Int. J. Curr. Microbiol. App. Sci. 5, 227–234.
25 Sempértegui, F., Díaz, M., Mejía, R., Rodríguez-Mora, O. G., Rentería, E., Guarderas, C., et al. (2007) Low concentrations of zinc in gastric mucosa are associated with increased severity of Helicobacter pylori-induced inflammation. Helicobacter, John Wiley & Sons, Ltd 12, 43–48
26 Mahmoud, A., Abuelazm, M., Ahmed, A. A. S., Abdalshafy, H., Abdelazeem, B. and Brašić, J. R. (2022) Efficacy and safety of polaprezinc-based therapy versus the standard triple therapy for eradication: A systematic review and meta-analysis of randomized controlled trials. Nutrients 14
27 Ibrahim, N., El Said, H. and Choukair, A. (2022) Zinc carnosine-based modified bismuth quadruple therapy standard triple therapy for eradication: A randomized controlled study. World J Clin Cases 10, 227–235
28 McEldrew, E. P., Lopez, M. J. and Milstein, H. (2025) Vitamin A. In StatPearls [Internet], StatPearls Publishing
29 Iyer, N., Grizotte-Lake, M., Duncan, K., Gordon, S. R., Palmer, A. C. S., Calvin, C., et al. (2020) Epithelium intrinsic vitamin A signaling co-ordinates pathogen clearance in the gut via IL-18. PLoS Pathogens 16, e1008360
30 Fan, X., Liu, S., Liu, G., Zhao, J., Jiao, H., Wang, X., et al. (2015) Vitamin A deficiency impairs mucin expression and suppresses the mucosal immune function of the respiratory tract in chicks. PLoS ONE 10, e0139131
31 Iyer, N. and Vaishnava, S. (2019) Vitamin A at the interface of host-commensal-pathogen interactions. PLoS Pathog 15, e1007750
32 Kearns, M. D., Alvarez, J. A., Seidel, N. and Tangpricha, V. (2015) Impact of vitamin D on infectious disease. Am J Med Sci 349, 245–262
33 Zhang, Y., Wang, C., Zhang, L., Yu, J., Yuan, W. and Li, L. (2022) Vitamin D3 eradicates Helicobacter pylori by inducing VDR-CAMP signaling. Front. Microbiol., Frontiers 13, 1033201
34 Institute of Medicine (US) Panel on Micronutrients. (2001) Molybdenum. In dietary reference intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US)
35 Grech, B. J. (2022) Dietary molybdenum may stimulate the growth of colonic sulfur reducing bacteria, increasing hydrogen sulfide levels in the human colon and the possible health effects of an excess of colonic sulfides. Arch Clin Gastroenterol 8 (2), 029-035.
36 Deng, K., Wang, L., Nguyen, S. M., Shrubsole, M. J., Cai, Q., Lipworth, L., et al. (2025) A dietary pattern promoting gut sulfur metabolism is associated with increased mortality and altered circulating metabolites in low-income American adults. eBioMedicine 115, 105690
37 Díaz-Gómez, M.F. & Hernández-Rosales, F. (2023) Can systemic parenteral ozone therapy generate biological ozone? A new hypothesis. Advances in Redox Research, Elsevier 7, 100063
38 Skrotzki, E. A., Vandavasi, J. K. and Newman, S. G. (2021) Ozone-mediated amine oxidation and beyond: A solvent-free, flow-chemistry approach. The Journal of Organic Chemistry, American Chemical Society
39 Aziz, N. and Jamil, R. T. (2023) Biochemistry, Xanthine Oxidase. In StatPearls [Internet], StatPearls Publishing
40 Montefiori, M., Jørgensen, F. S. and Olsen, L. (2017) Aldehyde Oxidase: Reaction Mechanism and Prediction of Site of Metabolism. American Chemical Society
41 Murali, M. R., Naveen, S. V., Son, C. G. and Raghavendran, H. R. B. (2014) Current knowledge on alleviating infections through the use of some commonly known natural products: bench to bedside. Integr Med Res 3, 111–118
42 Fahey, J. W., Stephenson, K. K. and Wallace, A. J. (2015) Dietary amelioration of Helicobacter infection. Nutr Res 35, 461–473
43 Rahnama, M., Mehrabani, D., Japoni, S., Edjtehadi, M. and Saberi Firoozi, M. (2013) The healing effect of licorice (Glycyrrhiza glabra) on Helicobacter pylori infected peptic ulcers. J Res Med Sci 18, 532–533
44 Sivam, G. P. (2001) Protection against Helicobacter pylori and Other Bacterial Infections by Garlic.The Journal of Nutrition, Elsevier 131, 1106S–1108S

.svg)





